By Claire Swedberg
Source: RFID Journal,
CDM has implemented an innovative technology-driven program to track dental instruments, believed to be the first of its kind among U.S. dental schools and providing multiple benefits including cost savings and improved inventory management. Utilizing radio-frequency identification (RFID) technology, the College is tracking 10,000 dental instruments using metal RFID tags developed by Xerafy, a Hong Kong-based producer which is an innovator in metal tag production and design. CDM implemented the program in June 2015.
Jan 12, 2016— When students attend the Columbia University College of Dental Medicine (CDM), they traditionally purchase their own dental instruments for use on patients at the school’s dental clinic, and are responsible for ensuring that those tools are not lost. Instruments commonly used include mirrors, probes and explorers, each of which is approximately 6 inches in length, cylindrical and composed mostly of steel. Tracking such items has been a difficult task for students, however, as well as for the clinic, which cleans and sterilizes them. Lost tools must be replaced, something that is costly for both the facility and the students.
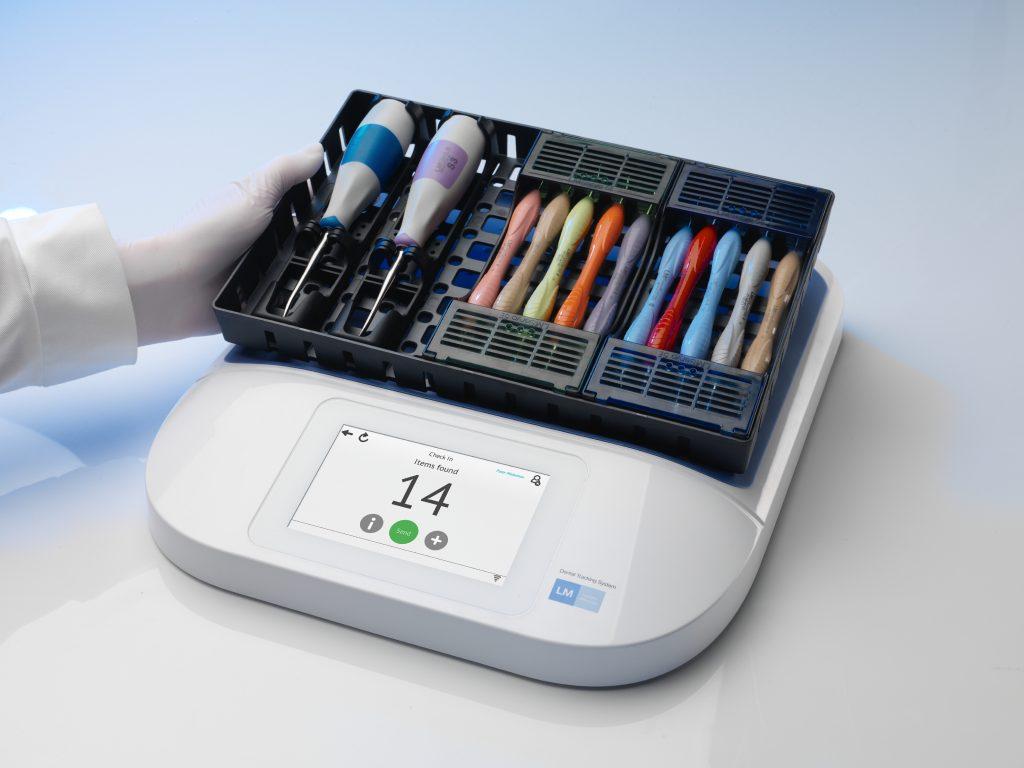
The solution adopted by CDM is a radio frequency identification system that tracks every instrument and the cassette in which it is packed, through clinical use, sterilization and storage. The technology, provided by dental instruments manufacturer LMDental, was taken live in June 2015. The majority of LM-Dental’s instruments come pre-tagged with autoclavable versions of Xerafy RFID tags attached to them, and have steel cores and silicone exteriors. CDM’s staff attach a Xerafy tag to any instruments used by the school that are not sold by LM-Dental. CDM has tagged 10,000 instruments to date, and expects to have all 20,000 items tagged by the end of this year.
The main driver for an RFID system, says Steven Erde, CDM’s chief information officer and an assistant professor of oral health informatics, was to identify a better method of documenting each tool’s use and sterilization for improved patient safety, and of meeting regulatory requirements. The management of dental instruments, however, is more complicated at a school with hundreds of students than in a small dental office.
The challenge for the 150-chair clinic, as well as for dental students (approximately 80 new students arrive each year), is to track tools not only when they are in students’ custody, but also when they are in the hands of the clinic’s own personnel, for sterilization by means of an autoclave. Before the RFID system was deployed, each student had to purchase a large set of instruments for all potential procedures he or she might encounter during training, loaded into 14 cassettes to form kits. These cassettes are composed of a mixture of plastic and stainless steel, and vary in size from 14 inches by 6 inches by 1 inch to 6 inches by 2 inches by 2 inches. The students kept cassettes packed with tools in a central storage area until using them, then turned them over to the clinic for sterilization and picked them up again when needed. At the time of cleaning and sterilization, employees at the clinic did not check each cassette to ensure that it contained all expected instruments. As such, students were responsible for tracking that information themselves, by visually identifying what was in every cassette.
The solution consists of an RFID tag on each instrument, linked to data about that asset in LM-Dental’s Dental Tracking System software, residing on CDM’s server. By using RFID, CDM has been able to eliminate the system of providing cassettes to students that they must then be responsible for throughout their time in the program. Instead, kits remain in CDM’s ownership and are checked out when required, then are returned to the clinic for sterilization and are stored there until requested by another student.
To enable this process, CDM installed LM-Dental’s tabletop RFID readers at several key locations, says Steven Connor, CDM’s clinic associate director: at the point at which cassettes are checked out, as well as on two sides of the autoclave unit—where tools are placed prior to sterilization, and where the sterilized instruments are removed from the autoclave unit. In the future, he notes, readers will also be installed in storage areas to help the staff track any cassettes not in use.
LM-Dental chose autoclavable versions of Xerafy’s Dot XS and Dash XS ceramic RFID tags because no other manufacturers make similar tags that are small enough for the company’s purpose, according to Stephen van Heerden, the firm’s system sales manager. In addition, says Moses Chang, Xerafy’s sales and marketing manager, there are no other tags currently on the market that have been as thoroughly tested and validated, and that are as widely in use, as Xerafy’s autoclavable tags. “With the lack of real estate on the majority of surgical instruments today,” Chang says, “the challenge, from a technical standpoint, is to create a tag small enough as to not interfere with the use, but still provide enough performance to create real value for the customer.” Xerafy’s family of autoclavable tags, he adds, were specifically built with all of these challenges in mind.
LM-Dental’s Stephen van Heerden The idea of the RFID solution came, in part, from the College of Dental Medicine’s dean, Erde reports. That individual was technologically savvy and interested in finding a technological way to facilitate the tracking of tools. CDM met with LM-Dental, which had recently developed an RFID-based solution to manage the dental instruments it manufactures. CDM’s system was taken live last summer, after the school tested it for about nine months on a small number of tagged cassettes and instruments. When a student requires a cassette of tools for a scheduled procedure, that individual reports to the instrument checkout area and presents his or her student ID card to an HID RFID reader, which scans the card’s ID number, links that data to the student’s identity and forwards it to the LM-Dental software. An employee at the checkout window retrieves the specific cassette that the student requires and places it on an LM-Dental EPC ultrahigh-frequency (UHF) RFID reader, which then links the tag IDs of the student and the kit.
The student takes the kit, uses the instruments for the patient procedure and then returns the cassette, with the dirty tools inside it, for cleaning and sterilization. Once the items are cleaned, they are placed on the reader at the autoclave’s entrance door The system interrogates all tags and confirms that all instruments are present. If any are missing, CDM can contact the student to determine if he or she can find them.
The cassette and instrument tags are read again after sterilization is complete, at the autoclave unit’s exit door. The statuses of the tools and cassette are then updated as cleaned, sterilized and ready for reuse.
According to Erde, the clinic sterilizes as many as 200 to 300 kits daily and finds about a dozen with problems, such as a missing instrument. That number of problems has decreased since the RFID system’s implementation, he says, in part because students feel more accountable regarding each tool.
Additionally, the clinic now has an automated record of when each tool was checked out for use with a particular patient, and when it was returned for sterilization and cleaning. This data also enables the facility to better manage when the instruments are sent out for sharpening, based on the amount of use.
The next phase of the deployment will be to expand the system to track the kits within three storage areas. A worker at each location would use an RFID reader to capture the tag ID of each loaded cassette as that individual places the cassette into or removes it from storage. CDM is also designing a larger dental clinic with 50 new chairs, and the RFID solution will be adopted for tools used at this new facility as well.
“We’re looking into tracking instrument utilization at the chair,” Erde states. If a student picks up a tool, software would detect its utilization based on the distance of its tag’s transmission to a reader, and would thus determine that the tool was used on a patient. The instrument’s use would be tracked so that the clinic could better understand which assets are heavily used, and which are not and might not be needed.
“We’re looking into tracking instrument utilization at the chair,” Erde states. If a student picks up a tool, software would detect its utilization based on the distance of its tag’s transmission to a reader, and would thus determine that the tool was used on a patient. The instrument’s use would be tracked so that the clinic could better understand which assets are heavily used, and which are not and might not be needed. “Additionally,” Erde says, “we can look for patterns in utilization by students that might effect outcomes of treatment, and proactively identify and work with students to improve their technique.”
According to Erde, the clinic also hopes to begin tracking patients via RFID wristbands and readers, to better understand how quickly patients are moving through their visit to the facility. Which readers would be employed for such use cases has yet to be decided.
Although the software resides on a hosted cloud-based server, Erde reports, it will be integrated with CDM’s own software on its own server in the future.
A third phase of the deployment may involve RFID-enabled cabinets that would automatically monitor the presence of each cassette and instrument in real time, until they are removed from the cabinet.
LM-Dental had been developing the solution for approximately the past three and a half years, van Heerden says, and is now marketing its Dental Tracking System to dental offices and schools. The solution comes with two tagging options: For the hand instruments that LM-Dental manufactures, the company embeds the RFID tags (such as Xerafy’s autoclavable tags) in the tools. For third-party instruments, the firm has developed a silicone ring that fits around each item in order to hold its RFID tag in place. “We believe that we have a very good and cost-effective solution for our hand instruments which does not alter the instrument at all,” he says, “but are continuously developing our tagging methods for third-party instruments to make it more ergonomic and cost-efficient.”
Because LM-Dental was unable to find readers that could effectively interrogate tags on tools, van Heerden reports, it designed its own. The software is cloud-based and was developed specifically for the dental industry, he says, adding, “but can also be modified for other medical industries.” The software allows users to perform customizable analytic reports and receive alerts based on customizable triggers.